|
On November 18, 2017, a research team led by Dr. Wanli Liu from School of Life Sciences, Tsinghua University published a research article entitled “A PIP2 derived amplification loop fuels the sustained initiation of B cell activation” in Science Immunology, which is a new Science family journal founded last year. In this study, the group reports the molecular mechanism explaining how the immune activation of B lymphocyte is precisely regulated by phosphatidylinositol 4, 5-biphosphate (PIP2). The results show that the amplification loop derived from the spatial and temporal hydrolysis and regeneration of PIP2 within the immunological synapse fuels the sustained initiation of B cell immune activation.
B lymphocytes, as important participants in the antibody response, are involved in maintaining human health. The immune activation of B cell is a key step in the initiation of humoral immune response. B cells are activated by the recognition of pathogen through the cell surface B cell receptor (BCR), with instantaneous and highly dynamic properties, which has been a hot spot in immunological research for twenty years. After BCR antigen recognition, BCRs oligomerize to form signaling microclusters and accumulate at the antigen interface to form immunological synapse, which functions as the platform for the continuous transmembrane signal transduction and antigen gathering. The antigens’ B cells encounter may be rare, and the physical and chemical properties are diverse, which requires B cells with efficient signal transmembrane transduction mechanisms and intracellular signal amplification mechanisms to promote immune activation. In addition, PIP2 is a rare phospholipid on the plasma membrane, the mechanism of their regeneration after being depleted and their effects on the activation of B cells remains unexplained. In this study, various lipid biosensors, genetically modified cell lines, mouse and human primary B cells and PLCγ2 mutants from PLCγ2-associated immunodeficiency and dysfunction (PLAID) disease, combined with total internal reflection and confocal fluorescence microscopy based high-speed and high-resolution imaging systems were used to uncover the mechanism underlying the spatial and temporal metabolic regulation of PIP2 and its effects on B lymphocyte activation.
This study identified a signal amplification mechanism derived from PIP2 that is responsible for the sustained activation of B cells. After antigen recognition, phospholipase Cγ2 (PLCγ2) hydrolyzes PIP2 inside the BCR microclusters, and phosphatidylinositol 4-phosphate 5-kinase (PIP5K) catalyzes PIP2 regeneration outside the BCR microclusters. The signal transduction from inside to outside of the BCR microclusters is achieved by the fast diffusion of diacylglycerol (DAG), produced by the hydrolysis of PIP2 inside the BCR microclusters into outside the BCR microclusters, followed by the conversion of DAG to phosphatidic acid (PA) catalyzed by diacylglycerol kinase ζ (DGKζ), PA can then recruit and stimulate PIP5K to regenerate PIP2. Both the low density of PIP2 inside the BCR microclusters and the high density of PIP2 outside the BCR microclusters are important for the initiation of B cell activation. Moreover, PLCγ2 mutants from PLAID patients result in aberrant PIP2-derived signal amplification loop in B cells. These finding provide new insights into immune recognition, activation and regulation for B lymphocytes and help in understanding the immunodeficiency-related diseases and also provide important information on the pathogenesis of related diseases, which may lead to development of improved vaccines and drugs.
Dr. Wanli Liu’s group is committed to investigate the function of lymphocytes by combining immunological and biochemical experimental approaches with cutting-edge high resolution high speed live cell and molecular imaging techniques along with biophysical research methods. Following the publications in the Nature Communications and eLife in 2015, Journal of Experimental Medicine and PNAS in 2016, eLife and Cell Reports in 2017, and The Journal of Immunology (2013, 2014, 2017) about immune activation of B lymphocytes, this research is his latest contribution to this field.
Chenguang Xu, a Ph.D. student from School of Life Sciences, Tsinghua University, is the first author, and Dr. Wanli Liu is the corresponding author of this article. This study required a strong integration of molecular biology, cell biology, biochemistry, high-resolution live cell imaging, biophysics as well as other disciplines of cross-advantages. During the course of the research, they were supported by Professor Chenqi Xu from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Professor Hai Qi from School of Medicine, Tsinghua University, Professor Lei Liu from Department of Chemistry, Tsinghua University, and Professor Haipeng Gong from School of Life Sciences, Tsinghua University. The study is funded by the National Science Foundation of China, the Ministry of Science and Technology of People's Republic of China, Young Thousands Program and Institute of Immunology, Tsinghua University.
Link of the paper: http://immunology.sciencemag.org/content/2/17/eaan0787
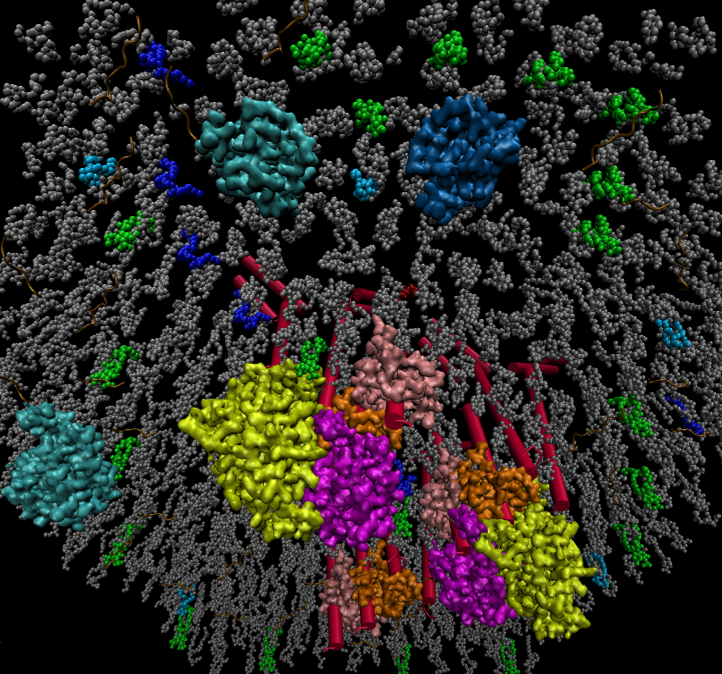
Figure. The positive feedback loop derived from PIP2 hydrolysis and regenerative metabolism enhances transmembrane signal transduction and immune activation of B lymphocytes. The perspective of this figure shows in the cytoplasm up looking at the plasma membrane. PIP2 (green), DAG (blue), PA (light blue), other membrane lipids (gray), DGKζ (cyan), PIP5Kα (indigo), BCR (red), Lyn (pink), Syk (orange), Blnk (purple), PLCγ2 (yellow).
|
